For an extra credit assignment Mr. Wong told us to identify electrolytes in my fridge! Here my fridge is labeled and divided into 6th different sections with each having different types of foods.
1st. Milk contains many electrolytes like sodium, potassium, magnesium, and mainly calcium which I typically enjoy with cereal.
2nd. Eggs are a great source of protein as well as electrolytes. Eggs have an abundance of electrolytes in them. Calcium is one of those electrolytes.
3rd. Vegetables of all sorts have electrolytes. Veggies are a great source of electrolytes.
4th. Yogurts and cream cheeses defiantly contain electrolytes such as calcium because milk is a key ingredient within these products.
5th. Juice, specifically orange juice must contain a decent amount of electrolytes for it is made with fruit. Potassium is very common in orange juice as well as vitamin C.
6th. Here we have some Salmon and assorted cheeses. Both have great amounts of electrolytes.
Wednesday, December 13, 2017
Alternate Penny Battery EC - Lawton Corrigan
Yesterday during lunch Max Lux, Justin Le, and I attempted to build a penny battery for the second time. Unfortunately we were not able to have our LED produce light. The first time I tried in 6th period I was also unsuccessful (See previous blog entry). Like last time, we started by sanding 4 pre-1982 pennies in order to expose the zinc and copper. We then stacked the pennies in between solution soaked cardboard squares. The solution was of the vinegar and water variety. To see if we had made successful battery we held an LED to both sides and we unhappy when it did not light up. We attempted using larger pieces or cardboard in a last ditch effort, but still were without a working battery. Maybe if we could somehow have gotten more electrolytes in the battery it may have had more success.
Above are images or our materials. Bottom, is a picture of our pennies and cardboard pieces stacked.
Above is our failed attempt at using larger pieces of cardboard, As you can see the LED did not light up :(.
The electrolytes present in our battery are sodium Chloride. We thought that the energy from these electrolytes passed though our device and the led would uses that energy to light up, which it did not. In conclusion I think we tried our best on two attempts, meaning that this lab most likely is some what faulty or takes extreme precision to pull off.
Above are images or our materials. Bottom, is a picture of our pennies and cardboard pieces stacked.
Above is our failed attempt at using larger pieces of cardboard, As you can see the LED did not light up :(.
The electrolytes present in our battery are sodium Chloride. We thought that the energy from these electrolytes passed though our device and the led would uses that energy to light up, which it did not. In conclusion I think we tried our best on two attempts, meaning that this lab most likely is some what faulty or takes extreme precision to pull off.
Monday, December 4, 2017
Thinking about electrolytes and conductivity - Lab - Corrigan
Last Friday my group and I tried making our own electrolyte battery out of batteries.
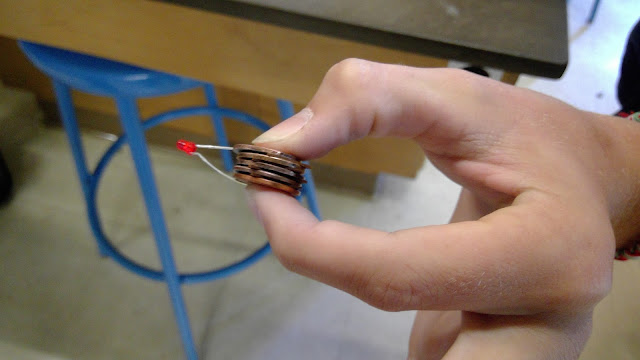
 Above is a picture of four pennies that we sanded down using sandpaper in order to see the zinc in these pre-982 pennies.
Above is a picture of four pennies that we sanded down using sandpaper in order to see the zinc in these pre-982 pennies.
 Above is a picture of four pennies that we sanded down using sandpaper in order to see the zinc in these pre-982 pennies.
Above is a picture of four pennies that we sanded down using sandpaper in order to see the zinc in these pre-982 pennies.
Next we doused our pennies and cardboard cut out square into a vinegar, salt, and water concoction.
Here is our final product which failed to light the led light (red). We made this by stacking the wet pennies and cardboard in a certain order.
Finally we tested the levels of electricity in our battery and found it had at least some charge. (see number in device).
The electrolytes present in our battery is sodium Chloride. We thought that the energy from these electrolytes passed though our device and the led would uses that energy to light up, which it did not. In the end, I think if we tried again we might be able to get it to work. This time might have been a little mistake somewhere in the process or just random bad luck.
Tuesday, November 14, 2017
Wednesday, November 8, 2017
Mendalien Info graphic - Corrigan
Today in Mr. Wong's class we needed to quickly come up with a Mendailen info graphic for Mr. Wong to present above is my graphic and below is my explanation of it! I chose to make the red arrow weight gain for the aliens eat more and gain more weight because of it. This is same as the black arrow because as you go across the aliens eat more and become fatter. The orange arrow represents increased sadness or loss of happiness because of hair loss, the opposite goes for the blue arrow. When these aliens loose hair and go bald they become sad. The brown and yellow arrows represent the sexual attraction gained or lost by aliens due to finger count. The green and purple arrows represent how well the aliens do in school based on number of arms. The more arms, obviously the easier work is to get done.
Sunday, November 5, 2017
Mundailens! - Lawton Corrigan
Today in Mr. Wong's 6th period chemistry class, we searched for the missing Mendailen! Below are my findings.
Thursday, October 26, 2017
Problem-solving lab: Interpret Scientific lustrations - Lawton Corrigan
In Mr. Wong's class, our most recent lab was to interpreted scientific illustrations and some of our own in the form of popular internet "memes." Here are two my classic memes about the Rydberg Constant (below).
Monday, October 23, 2017
mini-lab: Identify Compounds _ Lawton Corrigan
Today in Mr. Wong's chemistry class we got to try and figure out what substances we were burning based on the color of the flame, here are my guesses and pictures:






After these elements were burned, we were asked to analyze the colors and attempt to identify the element. Here is what we thought:
Substance #1 when heated showed a orange greenish flame at first, which turned into a bluish green flame after a few seconds.
Substance #2 above gave of a bright orange color.
When burning substance #3 (above) it also gave off a distinct orange coloration.
Substance #4 (above) was interesting because it gave a greenish, reddish, and even purplish color.
Substance #5 shown above had an reddish orange coloration.
Finally, substance #6 was pretty red for a decent amount of time before slowly turning into a more orange green combination.
Lab questions:
Think Critically: How can the single electron in a hydrogen atom produce all of the lines found in its emission spectrum?
When an electron becomes excited, in this case because of the heat energy, the electron travels across levels. This is a more up and down pattern which produces various coloration at different levels or heights.
Predict: How can you predict the absorption spectrum of a solution by looking at its color?
You can predict that the absorption spectrum of a solution by seeing where the substance will peak at the wavelength of the complimentary color of the solution.
Apply: How can spectra be used to identify the presence of specific elements in a substance?
A spectra can be used to identify the presence of specific elements in a substance because each element will produce unique colors and variations when exited with energy like heat. Those colors when observed, will help you uncover the the identity of the unknown substance.
Error Analysis. Name a potential source of error in this experiment. Choose one of the elements you observed, and research its absorption spectrum. Compare your findings with the results of your experiment.
A major potential source of error which definitely affected my group was cross contamination of the substances on the skewer which caused similarities in the color differentiation of the flame when the substances were burnt. This made the substances sometimes indistinguishable from one another.
Subscribe to:
Posts (Atom)