 Above is a picture of four pennies that we sanded down using sandpaper in order to see the zinc in these pre-982 pennies.
Above is a picture of four pennies that we sanded down using sandpaper in order to see the zinc in these pre-982 pennies.
Next we doused our pennies and cardboard cut out square into a vinegar, salt, and water concoction.
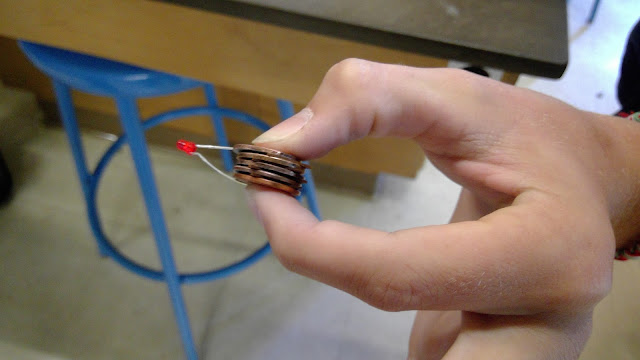
Here is our final product which failed to light the led light (red). We made this by stacking the wet pennies and cardboard in a certain order.
Finally we tested the levels of electricity in our battery and found it had at least some charge. (see number in device).
The electrolytes present in our battery is sodium Chloride. We thought that the energy from these electrolytes passed though our device and the led would uses that energy to light up, which it did not. In the end, I think if we tried again we might be able to get it to work. This time might have been a little mistake somewhere in the process or just random bad luck.


No comments:
Post a Comment